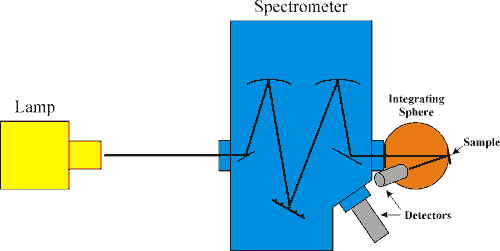
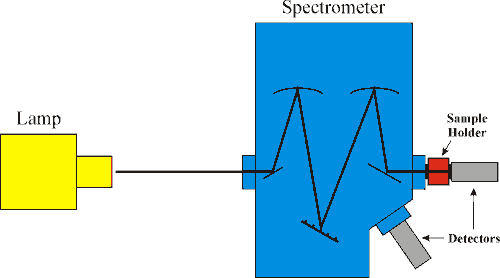
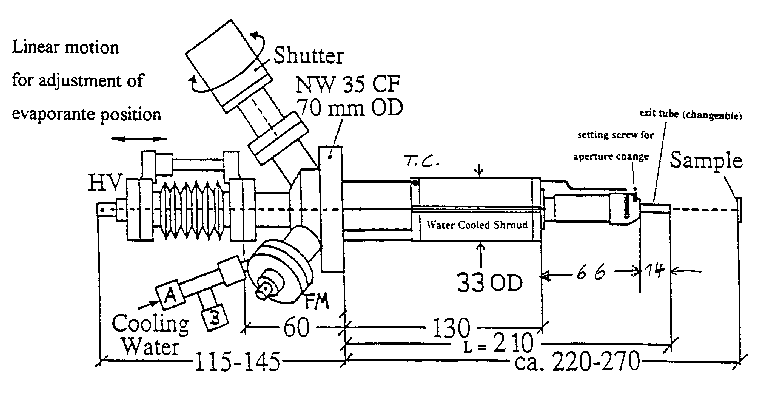
Pumping Components
The pumping action of a sorption pump is achieved by the cooling of a substrate material; this causes the air particles to stick to the substrate (this is similar to water vapor in the air condensing on a can of cold pop). The sorption pump is the roughing pump for the system, that is it takes the vacuum chamber from atmospheric pressures down to a low vacuum (in our lab we generally measure pressures in the units of Torr; atmospheric pressure is 760 Torr, and a low vacuum is around 10-3 Torr). An ion pump works by ionizing gas particles, and having those particles impinge into the walls of cathodes. An additional pumping process that occurs in an ion pump is that after the ionized particles hit the cathodes, some of the cathode atoms are discharged from the surface, and these deposit on all exposed surfaces of the pump. This is good because reactive gas particles will chemically attach themselves to the newly deposited cathode atoms. The ion pump is able to bring the chamber to a very high vacuum (10-8 Torr). The sublimation pump assists the ion pump, and it works in much the same way as the additional pumping process of the ion pump. That is, the sublimation pump evaporates titanium from a filament that gets deposited on the inside surface of the pump; this titanium is then exposed to the reactive gases inside the chamber, and it pumps out the reactive gases by chemically combining with them. The sublimation pump should be able to bring the chamber to ultra high vacuum levels (10-10 Torr). Even with these pumps, water vapor and some other gases are difficult to remove from the system. To remedy this problem we bake out the system (while the pumps are running, the chamber is brought to an elvated temperature causing the trapped gases to evaporate from the walls), and a cold trap is used (the cold trap works by having liquid nitrogen run through tubes inside the chamber, which causes the gas particles to freeze to the coils).
Presure Measuring Devices
The pressures inside the chamber are measured by two different instruments: an thermocouple gauge and an ionization gauge. A thermocouple gauge works by the gauge heating itself up, and then the rate of the temperature change is measured. This relates to a pressure in that the higher the pressure the more air particles that are in the chamber and the more thermally conductive is the atmosphere inside the chamber, and thus the quicker the rate of change of the temperature of the gauge. A thermocouple gauge can measure pressures down to 10-3 Torr. An ionization gauge works by a filament being heated (like in a light bulb) which then gives off electrons. These electrons are then collected, but if there are any air particles in the way of the electrons' path, the electron is either deflected away from the collector of the gauge or the particle is ionized by the electron. This causes the current produced on the gauge's collector to be different than if the gauge was in its reference pressure, and there is a direct relationship between the pressure and the current. This gauge can read from 10-4 Torr down to 10-10 Torr.